|
Preliminary Results of Leg M40/4 --- 9.2.98
Microbial Activities and Populations in the upper sediment and sapropel layers
(J. Overmann, M. Coolen, A. Smock, H. Sass, H. Cypionka)
Bacterial activities and populations were studied in the upper sediment and sapropel layers (down to S5). Main sites were station #69 (near ODP station 969) and the anoxic Urania basin (station #76). Oxygen penetration depths into the sediment and sulfide were measured with needle electrodes. Oxygen penetrated up to 30 cm at deep stations. Free sulfide was detected only in the Urania basin. The water in the anoxic brine beneath 3470 m contained enormous concentrations (up to 24 mM) of free H2S, the concentration in the underlying sediment was only 3 mM. Microbiological, molecular biological and geochemical approaches were used to analyze total and viable bacterial cell numbers, to measure the metabolism of radiolabelled and fluorescently labelled substrates, and to isolate intracellular and extracellular DNA. Significant cell numbers and activities were found even in the deepest samples analyzed, which have an age of about 120 ka. The samples will be used to compare the species composition and the physiological activities in the different layers and sampling sites. Additonal information about the bacterial metabolism will be obtained from the analysis of the 34S and 13C isotope signatures. We hope to contribute to the understanding of the origin and early diagenesis of the Mediterranean sapropels.
Methods
Oxygen Oxygen profiles were measured by means of two needle electrodes (Microscale Measurements, Den Haag, NL) with a diameter of 1 mm and an length of 15 and 30 cm, respectively. Calibration was performed with air-saturated seawater at 20 °C.
Sulfide Sulfide was measured on bord by means of a needle electrode (Microscale Measurements, Den Haag, NL) of 1 mm diameter and a length of 15 cm. Calibration was performed with sodium sulfide solutions under N2 in seawater buffered at pH 7 or pH 9 (Cypionka 1994). Furthermore, samples treated with zinc acetate were prepared for the spectrophotometric sulfide analysis according to Cline (1969).
pH and temperature pH and temperature of the water in multicorer cores, and the pH in the sediment (in 2 cm depth) were measured with a glass electrode (Ingold) and a resistance thermometer (WTW, Weilheim), respectively.
CTD A CTD profile, providing additional fluorescence and oxygen signals, was recorded down to 3520 m at the Urania Basin (Station #76), and water samples were taken with 16 Niskin bottles at depths between 3400 and 3520 m.
Sediment cores A gravity core was obtained from station #69 (near ODP station 969). Sediments from the anoxic Urania basin (station #76) were sampled with the multicorer. In addition we used samples of sapropel S3 from gravity cores #66-4 and #76-6 for several control experiments during the determination of bacterial activity (glucose oxidation and ectoenzyme activity, see below). Sample preparation To obtain contamination-free sediment samples, gravity cores were cut by half. The surface was covered by Seran wrap, and a 1 cm-thick layer of dry ice. After incubation for 5 min the Seran wrap was removed and the frozen layer beneath could be carefully lifted with sterile forceps in one piece, thereby leaving a freshly broken aseptic surface.
Bacterial counts Total numbers of bacteria were obtained by epifluorescence microscopy after staining with acridine orange. The most probable number (MPN) technique on microtiter plates was used to determine the number of viable anaerobic bacteria. Thirteen different media were used. In addition, agar plates for viable cell counts of aerobic heterotrophic bacteria were inoculated. For most media, parallel samples were pasteurized (10 min at 80 °C) in order to determine the fraction of spore-forming bacteria. Dilutions were performed on bord in an AtmosBag (Aldrich, Milwaukee, Wisconsin) gassed with argon.
Microbial activities Bacterial activities in sapropels and intermediate sediment layers were investigated by incubation with uniformely labelled 14C-glucose and 14C-acetate. The 14CO2 released during microbial degradation of glucose was trapped and quantified by liquid scintillation counting. The determination of 14CO2 formed from acetate will be quantified by HPLC in the home laboratory. The activities of the microbial ectoenzymes ß-glucosidase, protease, and alkaline phosphatase were assessed employing methylumbelliferyl- and aminomethylcoumarine-labelled fluorescent substrate analogs. All incubations were initiated within 24 h after sampling.
DNA extraction Sediment samples for the extraction of genomic DNA were obtained under aseptic conditions and immediately frozen until later analysis. For the extraction of extracellular DNA, a second set of samples was instantaneously mixed with a mixture of PVP/Tween 80 and embedded in sterile 1.5 % Agarose/TAE solution. These agarose blocks were subjected to electrophoresis (12 h, 100 V) in a Biotrap apparatus (Schleicher & Schüll) fixed in a custom-made cardanian suspension frame. The latter maintained a constant horizontal position of the gel apparatus despite of considerable movement of the research vessel. Afterwards DNA eluates were recovered from the trap chambers of the device and frozen at -20°C. Microbial biomass in water samples from the chemocline of the Urania basin was concentrated on 0.2 µm pore size polycarbonate filters and frozen for analysis of bacterial DNA.
Further sampling From multicorer cores, sediment surface samples and porewater (squeezed by means a home-made press at pressures of up to 5 bar), were collected, partially treated with zinc acetate and frozen for analysis of organic and inorganic carbon, sulfur compounds, and isotope signatures.
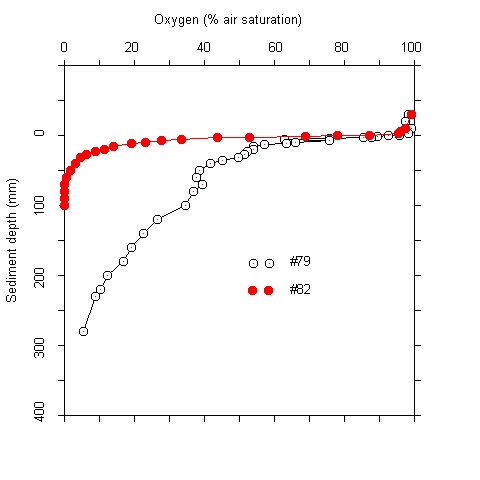
Results Oxygen penetration, pH, and sulfide concentrations at the sediment-water interface The oxygen concentrations at the sediment-water interface were near air saturation at most stations. Within the upper centimeter of the sediment the concentration decreased approximately by half (Tab. 1). The vertical extension of the oxic zone was 30 to a maximum of 280 mm. The highest values were found in sediments at water depths of more than 2400 m. Due to the asymptotic shape of the oxygen profiles, the exact extension of the oxic zone could not be defined in many cases (Fig. 1).
Tab. 1 Oxygen penetration, pH, and sulfide concentrations at the sediment-water interface
1) Minimum values. Due to an asymptotic approach to zero, the lower boundary could often notbe determined exactly.
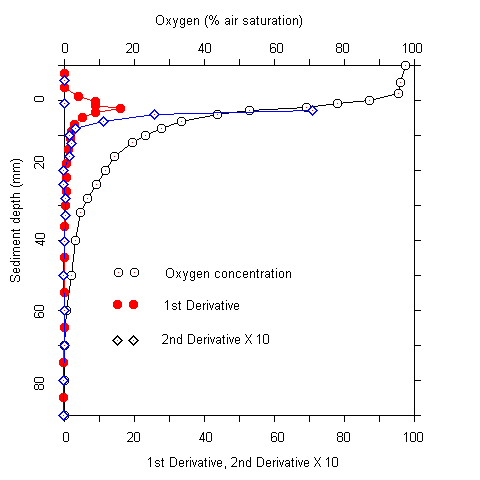
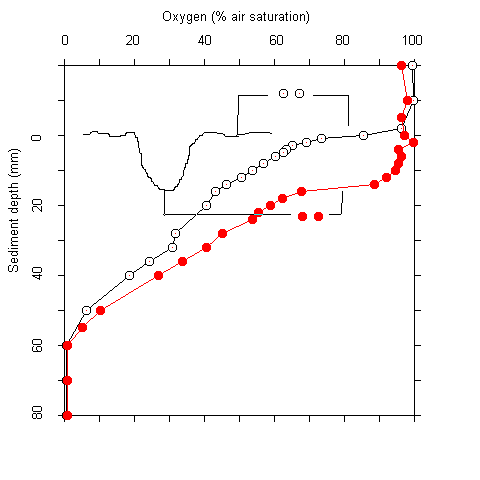
Plots of the first and second derivative of the oxygen profiles, which give hints on the oxygen flow and consumption rate indicated that most of the oxygen consumption took place in the upper millimeters of the sediment. Even where oxygen penetrated deeper than 5 cm, more than 90 % of the respiration acitivity was located in the upper 5 mm. The oxygen measurements were complicated by two facts. Firstly, there was bioturbation caused by macrofauna at several sites. This caused an increase of oxygen penetration around the channels formed (Fig. 2). Secondly, there was some scatter in the oxygen electrode signals. No noise was observed during measurements in the water column. In the upper sediment layers, however, the signal became noisy after about one minute. A possible explanation for this phenomenon is that the alkali produced at the electrode tip (O2 + 4 e- + 4 H+ ® 2 H2O) caused some precipitations at the electrode surface. These were wiped off when the electrode was moved A / B
Fig.1 Oxygen profiles at stations #79 and #82 (A). (B) Oxygen profile and first and second derivatives at station #85.
A B
Fig. 2 Core taken at station #78 showing macrofaunal bioturbation (A). (B) Oxygen penetration into the sediment within a borrow hole, and 1.5 cm off.
deeper to the next level. At low oxygen concentrations (and corresponding low alkali production by the electrode) the signal stayed stable. The pH values measured in the water above the multicorer cores were 8.2 to 9.1, while in the upper sediment layers they were 0.2 to 1.2 units lower (Tab. 1). Two hours after sampling and after having measured the oxygen profile, the pH in the water was usually decreased by about 0.5 pH units, probably due to release of buffering compounds from the sediment. Free sulfide was not detected in any of the multicorer cores except those from the Urania basin. Thus, there was no oxygen-sulfide interface in the other sediments.
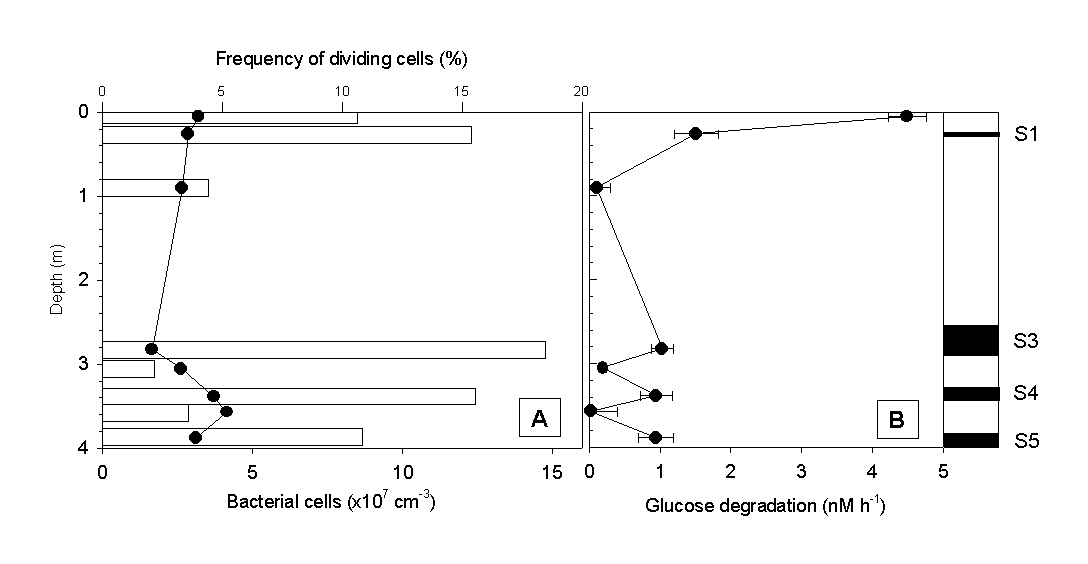
Microbiological analysis of sapropel layers and intermediate low-Corg layers Total numbers of bacterial cells reached values of about 108 per cm3 in all sapropel layers of the gravity core #69-2 (Fig. 3A). With the exception of the sediment surface, bacterial counts in layers of low Corg were much lower. No significant differences in the frequency of dividing cells was observed, however. A characteristic pattern of microbial activity emerged when the glucose degradation rates and ectoenzyme activities were determined. The highest rate of glucose mineralization was detected at the sediment surface. Below, significant microbial activity was detected in all 4 sapropel layers present, while the values in low-Corg intermediate layers were at or below the detection limit (Fig. 3B).
Fig. 3 (A) Total numbers of bacterial cells (bars), frequency of dividing cells (circles) and (B) glucose degradation rates in sapropels and intermediate layers of gravity core #69-2.
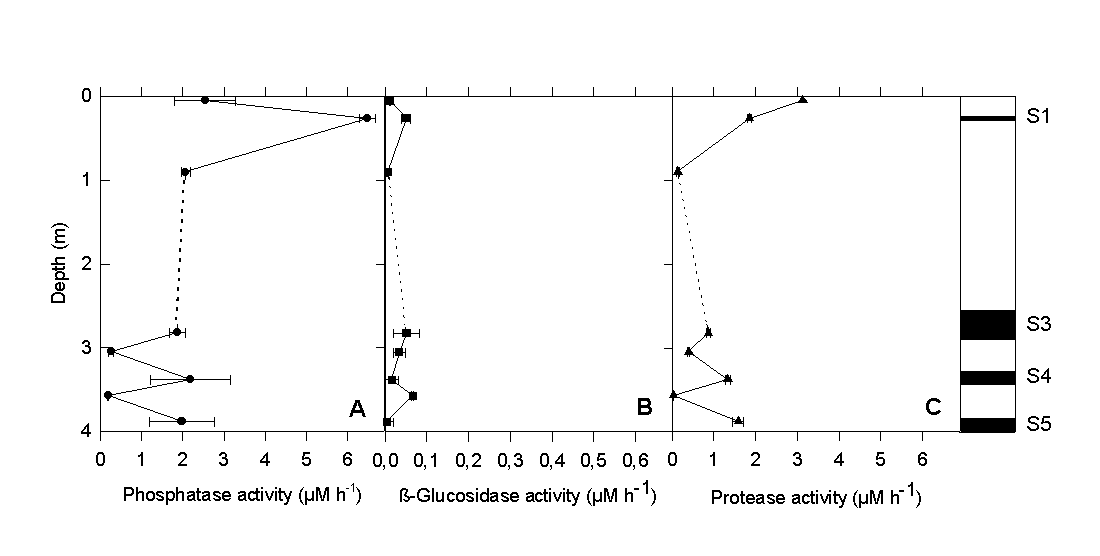
The vertical differences in protease activity (Fig. 4C) were very similar to those observed for glucose degradation. Phosphatase activity (Fig. 4A) reached maximum values in all sapropel layers. By comparison, the activity of ß-glucosidase (Fig. 4B) was one to two orders of magnitude lower and did not show the distinct vertical pattern of the other two ectoenzymes. Our experimental results clearly indicate that the sapropel layers harbor a microbial community which, despite of the high age of the surrounding sediment matrix, still exhibits measurable and significant physiological activity. The fact that rates were significantly lower in the sediment layers between the sapropels indicates that a contamination of the older layers by sediment from above did not occur during sample preparation. Because of the distinct maxima in bacterial cell numbers and physiological activity within the sapropels, a supply of carbon substrates solely by pore water transport appears unlikely. Rather, sapropels themselves must provide favorable conditions for the growth and survival of chemotrophic bacteria.
Fig. 4. (A) Ectoenzyme activity of alkaline phosphatase, (B) ß-glucosidase, and (C) protease in sapropels and intermediate layers of gravity core #69-2.
Geochemical and microbiological investigation of the Urania basin At station #76 a CTD profile was recorded down to 3520 m (Fig. 5A). Water samples above, in, and below the chemocline were obtained (Fig. 5B). Furthermore, sediment samples were taken from a multicorer.
A B
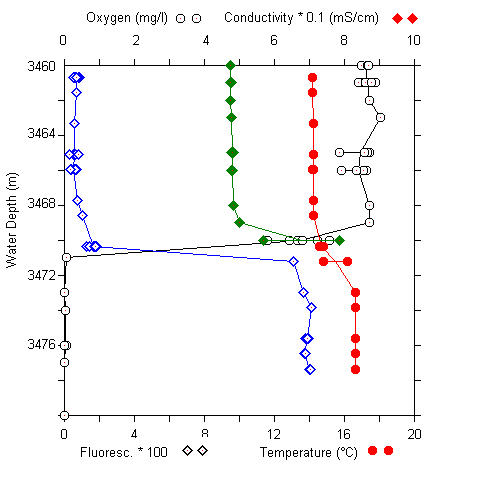
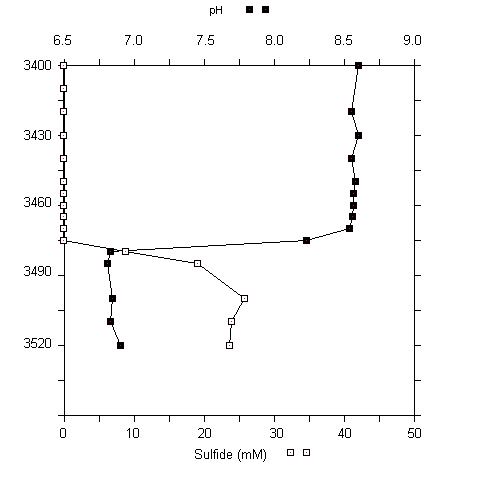
Fig. 5 (A) CTD profile at 3460 to 3480 m at the urania basin. (B) pH and sulfide concentrations in water samples (3400 to 3520 m).
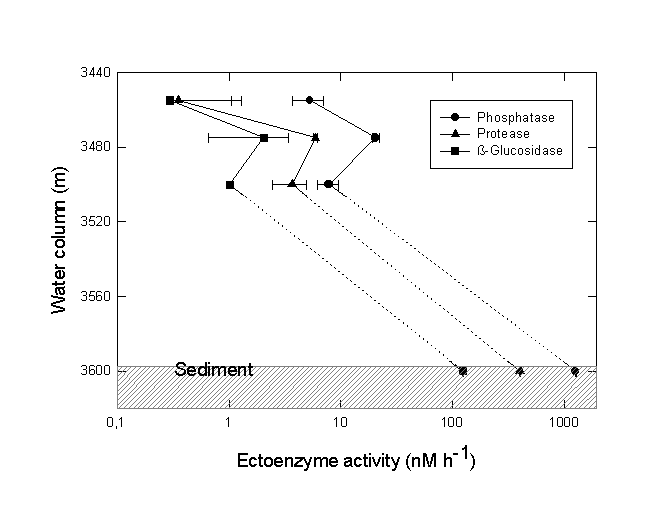
A sharp chemocline had already been detected at a water depth of 3470 m (MEDRIFF consortium 1995). The temperature increased by more than 2 °C within 2 m. Concomitantly, the oxygen concentration dropped to zero, and the fluorescence signal increased by a factor of ten. The conductivity record stopped at 58 mS/cm due to range overflow. In the water samples, a pH shift from 8.5 to 6.7 was found. Furthermore, free H2S was detected at concentrations of up to 24 mM. If the electrode data are verified by chemical analysis at home, our values would represent the highest free sulfide concentration ever found in seawater (Luther et al. 1990; Henneke et al. 1991). In the sediment only 3 mM free sulfide was detected. Within the free water column of the Urania deep, maximum activities of all three ectoenzymes were detected in the chemocline (Fig. 6), indicating a zone of increased bacterial activity at the oxic/anoxic interface. Activities of alkaline phosphatase and protease were lower in the surface sediment layer at this site compared to surface sediments in core #69-2. Similarly, the degradation rate of glucose was lower by one order of magnitude (0.362 nM Glucose h-1). In contrast, ß-glucosidase activity in Urania basin sediment was increased by a factor of 1000.
Fig. 6. Ectoenzyme activity of alkaline phosphatase, protease and ß-glucosidase in the free water column and surface sediment of the Urania basin. The chemocline was positioned at 3470 m depth. Note the logarithmic scale of enzyme activity values.
Conclusions and further investigations Our measurements on bord have revealed elevated bacterial cell numbers and microbial activity in up to 124,000 year old sapropel layers of the Mediterranean. In our forthcoming work we are going to analyze the bacterial communities by microbiological, molecular biological and geochemical approaches. We have the opportunities to compare sapropel layers of different ages with layers of low organic content. The anoxic water body in the unique Urania basin shows conditions that might be compared with those during development of the sapropels in some aspects. Furthermore, the sharp chemocline with extreme sulfide concentrations will probably harbour a community of sulfide-oxidizing bacteria. We are going to analyze the presence of those bacteria in the water and in the sediment, where the conditions do not allow growth by sulfide oxidation with O2. This might allow to differentiate between inactive bacteria from the water body and populations growing in the sediment.
• The molecular biological studies will include aseptical extraction of the total DNA, followed by PCR amplification of 16S rDNA sequences and denaturing gradient gel electrophoresis in order to to analyze the composition and diversity of the bacterial communities. PCR methods for the selective amplification of specific groups of bacteria will also be used. The free DNA extracted electrophoretically from a series of sediment samples will be used to differentiate between living bacteria and fossil residues. A similar approach will be used for the phylogenetic characterization of microbial communities present in the chemocline of the Urania basin, using the samples of bacterial cells concentrated on membrane filters.
• The microbiological investigations will be based on the isolation in pure culture of the bacteria present in the highest MPN dilutions and in the various enrichment cultures. The strains obtained will be compared phylogenetically with the composition of the whole bacterial community. Furthermore, a physiological characterization of bacterial strains will provide more insight into the culturability of deep-sea bacteria from ancient sediment layers. The 16S rDNA sequences of the isolates will be determined and compared with the sequences present in the total and free DNA extracts.
• The geochemical characterization will include a chemical analysis of inorganic sulfur and organic carbon compounds and an analysis of the stable isotopic compositions. This will hopefully more precisely determine the role of bacteria in the transformation of these compounds within the sediment layers. Our set of pore water samples will be used to determine low molecular weight compounds (carbohydrates, amino acids) present using high sensitivity HPLC techniques (PAD and OPA). The sulfur compounds to be analyzed chemically and isotopically include sulfate, FeS, FeS2, S0, H2S. Furthermore, bacterial membrane lipids and diagenetically altered lipids will be extracted and identified.
Literature
Cline JD (1969) Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 14:454-458
Cypionka H (1994) Sulfate transport. Meth. Enzymol. 243:3-14
Henneke E, Luther GW, De Lange GJ (1991) Determination of inorganic sulfur speciation with polarographic techniques: Some preliminary results for recent hypersaline anoxic sediments. Mar. Geol. 100:115-123
Luther GW, Catalano G, De Lange GJ, Woittiez JRW (1990) Reduced sulfur in the hypersaline anoxic basins of the Mediterranean Sea. Mar. Chem. 31:137-152
Overmann J (1996) Analysis of an ancient microbial community using molecular methods. PE 127, VAAM Jahrestagung Bayreuth 1996
Overmann J, Beatty JT, Krouse HR, Hall KJ (1996) The sulfur cycle in the chemocline of a meromictic salt lake. Limnol Oceanogr 41:147-156
MEDRIFF Consortium (1995) Three brine lakes discovered in the Seafloor of the Eastern Mediterranean. EOS 76:315-320
Rochelle PA, Fry JC, Parkes RJ, Weightman AJ (1992) DNA extraction for 16S rRNA gene analysis to determine genetic diversity in deep sediment communities. FEMS Microbiol. Lett. 100: 59-66
|